Allergic reactions to the first COVID‐19 vaccine: a potential role of polyethylene glycol?
Allergic reactions to the first COVID‐19 vaccine: a potential role of polyethylene glycol?
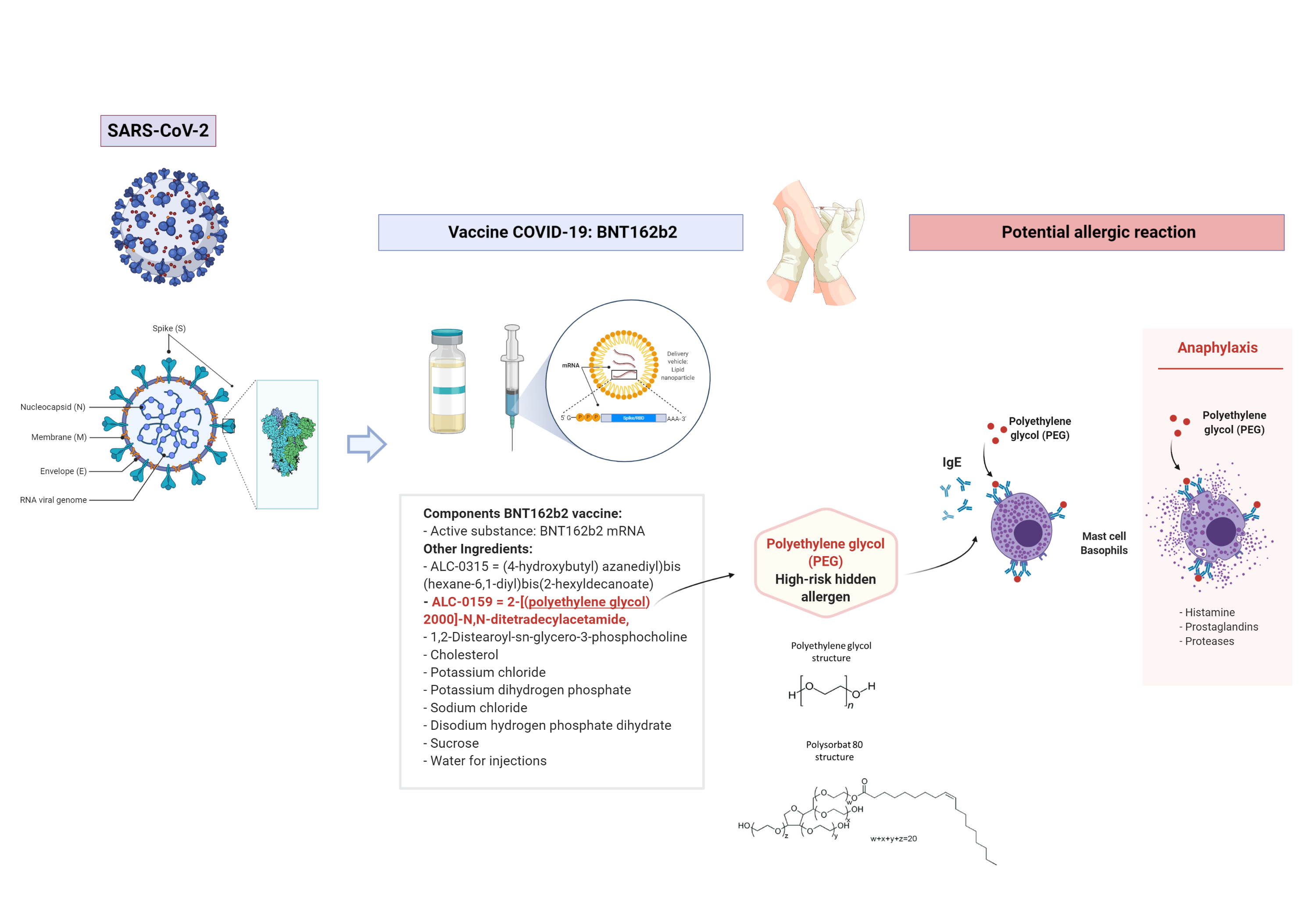
The COVID‐19 vaccine developed by Pfizer and BioNTech was approved by the Medicines and Healthcare Products Regulatory Agency (MHRA) in the United Kingdom (UK) on December 2nd 2020. MHRA is therefore the first regulator agency in the world to approve a vaccine to prevent coronavirus disease (COVID‐19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), a virus that is responsible for a global pandemic.