Skin tests in urticaria/angioedema and flushing to Pfizer‐BioNTech SARS‐CoV‐2 vaccine: limits of intradermal testing
Skin tests in urticaria/angioedema and flushing to Pfizer‐BioNTech SARS‐CoV‐2 vaccine: limits of intradermal testing
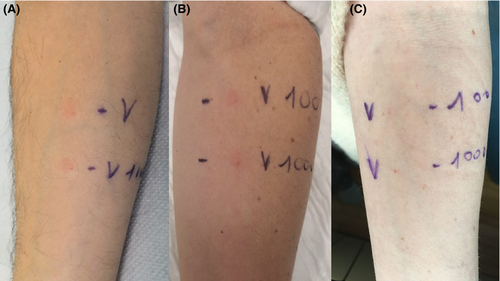
During clinical approval studies and early post‐marketing phases, mucous‐cutaneous adverse reactions have been rarely observed. Among hypersensitivity reactions, immediate reactions (anaphylaxis, urticaria‐angioedema syndrome) were more frequently observed than delayed reactions (maculo‐papular eruptions).